 Figure | The tire of life by Ferdinand Hodler illustrates the dramatic effects of severe depression on the mind and body
Figure | The tire of life by Ferdinand Hodler illustrates the dramatic effects of severe depression on the mind and body
The physiological basis for distributed neuronal network remodelling in chronic stress.
Chronic stress is an etiological factor supporting the induction of depressive symptoms. How does exposure to stress affects the brain and how to remedy it are important questions for the development of novel and effective therapies.
In the Netherlands, depression affects 8% of the population, and in extreme cases is associated with the ~1800 yearly suicides. Current anti-depressants are slow to act and will fail to work in a sizable population of treatment-resistant patients.
We have examined the brain-wide remodelling within and between distributed neuronal networks in a mouse model of psychosocial stress. The patterns observed in chronically stressed animals closely resembled those found in human patients using homologous methods, however, the underlying biological mechanisms behind this phenotype is not understood.
To causally evaluate the role of serotonin, a major neurotransmitter implicated in depression, we have leveraged a method to visualize whole-brain activity in response to serotonin release. We found that stressed animals had a reduced response to serotonin release, whereas animals receiving serotonin-reuptake inhibitors had the opposite response. We seek to implement this method to further our understanding of neuronal remodelling under acute and chronic stress, and to understand the role of serotonin in a variety of cognitive tasks.
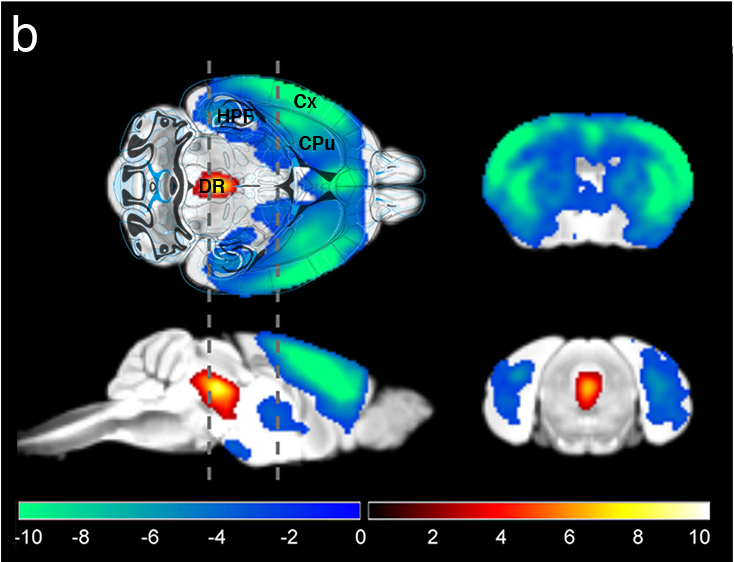 Figure | Visualization of the serotonin system with fMRI and optogenetics
Figure | Visualization of the serotonin system with fMRI and optogenetics
Serotonin-reuptake inhibitors are slow to act, and often fail to work altogether. Psilocybin, an agent derived from ‘magic mushroom’, is considered to exert rapid and potent anti-depressive properties. To better understand its role, we administered psilocybin in healthy mice during whole-brain recordings. We found that psilocybin acts on neuronal networks that were not identified in animals under-going chronic stress. The effect of psilocybin was found nearly exclusively in the ventral striatum, an area associated with reward and dopamine. This observation reconciles some of the conflicting findings in human, but suggest a mechanism for Psilocybin mode of action.
References
Grandjean, Joanes; Corcoba, Alberto; Kahn, Martin C; Upton, A Louise; Deneris, Evan S; Seifritz, Erich; Helmchen, Fritjof; Mann, Edward O; Rudin, Markus; Saab, Bechara J; A brain-wide functional map of the serotonergic responses to acute stress and fluoxetine Nature communications 10 1 350 2019
Grandjean, Joanes; Azzinnari, Damiano; Seuwen, Aline; Sigrist, Hannes; Seifritz, Erich; Pryce, Christopher R; Rudin, Markus; Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression Neuroimage 142 544-552 2016
Grandjean, Joanes; Buehlmann, David; Buerge, Michaela; Sigrist, Hannes; Seifritz, Erich; Vollenweider, Franz X; Pryce, Christopher R; Rudin, Markus; Psilocybin exerts distinct effects on resting state networks associated with serotonin and dopamine in mice bioRxiv 751255 2019
The early mechanisms leading to functional alterations in Alzheimer’s disease
Neurodegenerative disorders exert a huge toll on our ageing populations. The dramatic decline in cognitive function is distressing for both the patients and the families witnessing the disease.
No disease-modifying treatment exists for the disease. Moreover, beyond a genetic (e.g. APOE4 allele) and environmental (e.g. cardio-vascular) risk factors, the steps leading to the pathology remain elusive.
Using animal models and neuroimaging, we investigate the earlier stages of the pathology and its relation to brain structure and function.
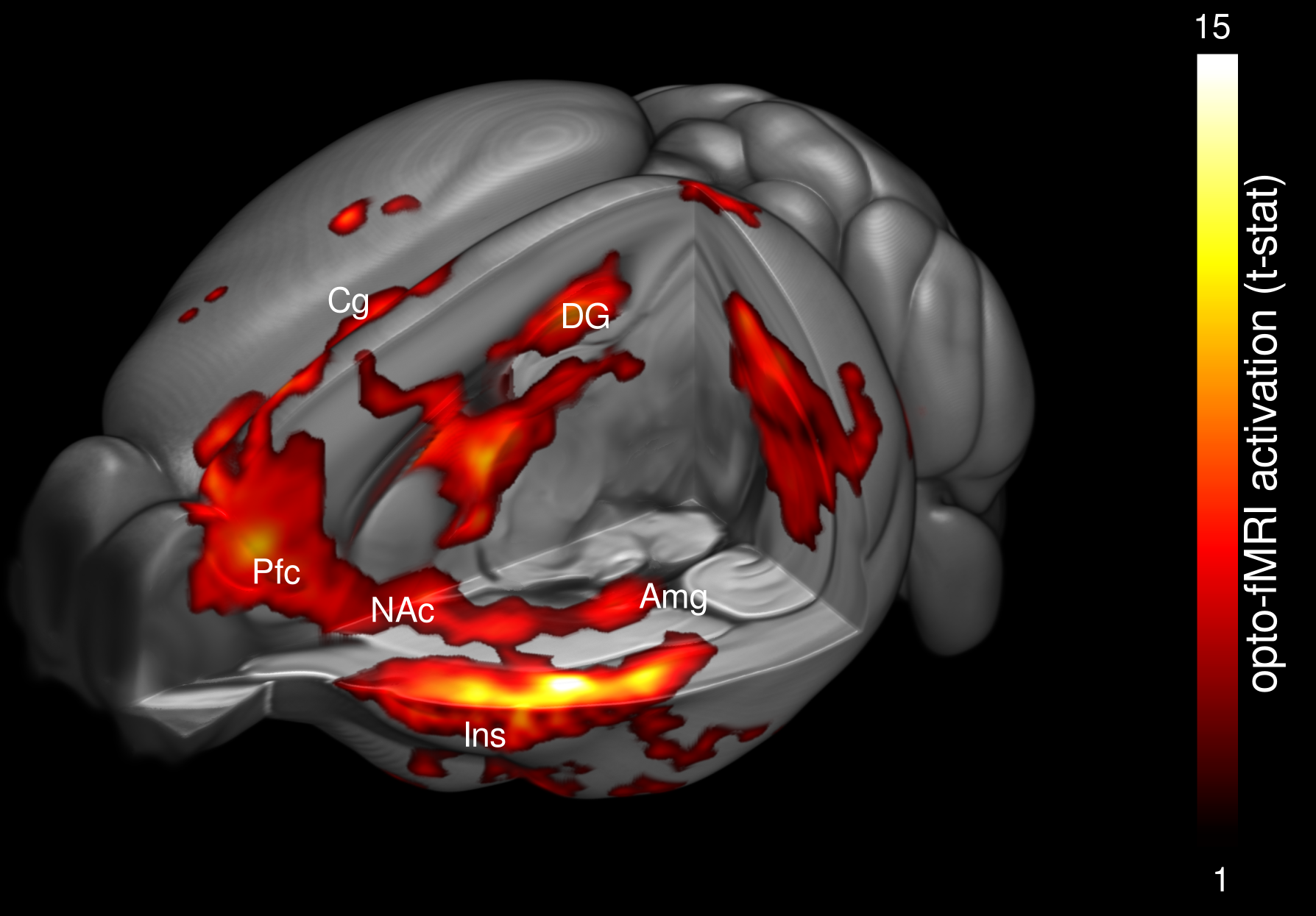 Figure | Visualization of the entorhinal connectivity with fMRI and optogenetics in a mouse model of Alzheimer’s disease
Figure | Visualization of the entorhinal connectivity with fMRI and optogenetics in a mouse model of Alzheimer’s disease
Using optogenetics and functional imaging, we have characterized a dichotomy between resting and evoked neuronal activation in the entorhinal cortex, a region strongly affected in Alzheimer’s disease.
References
Grandjean, Joanes; Derungs, Rebecca; Kulic, Luka; Welt, Tobias; Henkelman, Mark; Nitsch, Roger M; Rudin, Markus; Complex interplay between brain function and structure during cerebral amyloidosis in APP transgenic mouse strains revealed by multi-parametric MRI comparison Neuroimage 134 1-11 2016
Grandjean, Joanes; Schroeter, Aileen; He, Pan; Tanadini, Matteo; Keist, Ruth; Krstic, Dimitrije; Konietzko, Uwe; Klohs, Jan; Nitsch, Roger M; Rudin, Markus; Early alterations in functional connectivity and white matter structure in a transgenic mouse model of cerebral amyloidosis Journal of Neuroscience 34 41 13780-13789 2014
The physiological basis for neuronal network organization.
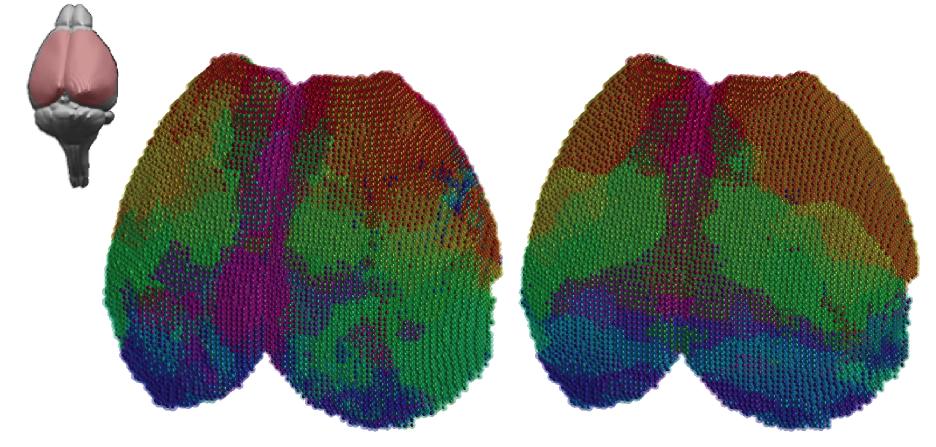 Figure | A comparison of structural and functional connectivity
Figure | A comparison of structural and functional connectivity
Functional neuroimaging methods are widely used in human to derive mechanistic information related to brain pathologies. However, experimental limitations imposed in humans preclude molecular and cellular analysis. Using trans-species imaging modalities, we investigate the physiological basis for functional connectivity phenomena in the healthy and diseased brain.
References
Grandjean, Joanes; Zerbi, Valerio; Balsters, Joshua Henk; Wenderoth, Nicole; Rudin, Markus; Structural basis of large-scale functional connectivity in the mouse Journal of Neuroscience 37 34 8092-8101 2017
Grandjean, Joanes; Preti, Maria Giulia; Bolton, Thomas AW; Buerge, Michaela; Seifritz, Erich; Pryce, Christopher R; Van De Ville, Dimitri; Rudin, Markus; Dynamic reorganization of intrinsic functional networks in the mouse brain NeuroImage 152 497-508 2017
Reproducible neuroscience.
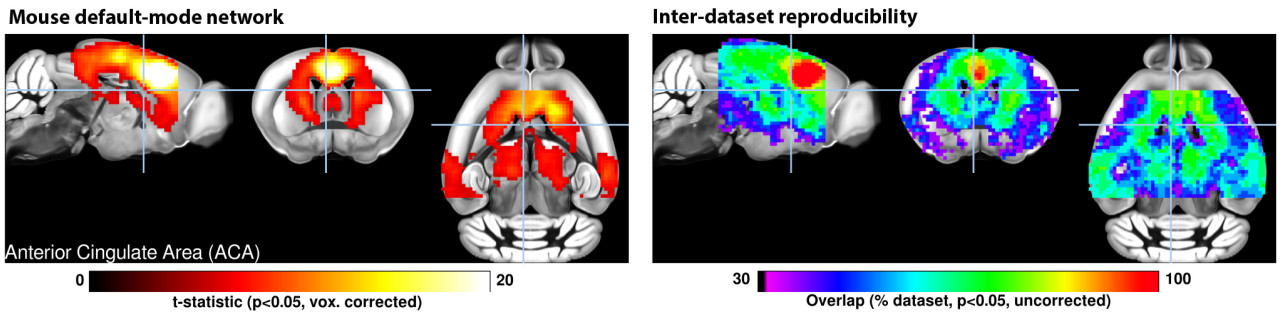 Figure | A mutli-center assesment of the mouse default mode network
Figure | A mutli-center assesment of the mouse default mode network
Science is a continually evolving system. Animal neuroimaging has developed rapidly over the past 10 years, fueled in part to functional imaging possibilities. We have been involved since the beginning in providing resources to help new groups implement the methods. Since 2014 we have made all of our dataset freely available online (see resources).
We have spearheaded the first animal fMRI multi-centre comparison study, and we now continue to provide resources to foster the development of animal fMRI. I am happy to review dataset from labs seeking to implement animal fMRI technologies.
References
Grandjean, Joanes; Canella, Carola; Anckaerts, Cynthia; Ayrancı, Gülebru; Bougacha, Salma; Bienert, Thomas; Buehlmann, David; Coletta, Ludovico; Gallino, Daniel; Gass, Natalia; Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis NeuroImage 205 116278 2020
Grandjean, Joanes; Schroeter, Aileen; Batata, Imene; Rudin, Markus; Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns Neuroimage 102 838-847 2014